Objective
The PROTECT trial will test the hypothesis that proton (PT) -enabled radiation dose reductions to sensitive, normal tissues will result in lower rates of treatment-related pulmonary complications in esophageal cancer compared to standard photon therapy (XT).
Methodology
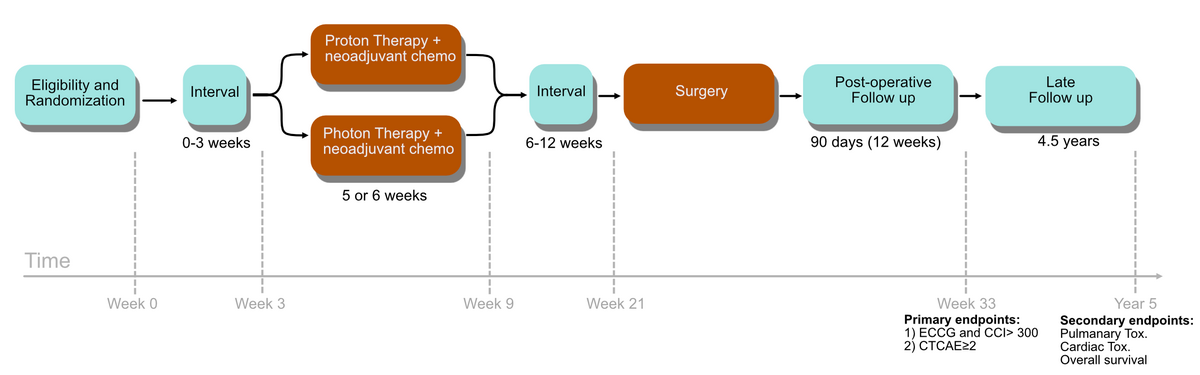
PROTECT is an open-label, non-blinded, international multicenter, randomized phase III study for patients with operable esophageal cancer (EC) or esophagogastric cancer (EGC) receiving neoadjuvant chemoradiotherapy (nCXT) (standard of care) or nCPT (intervention). Randomization is 1:1 to nCPT or nCXT. Stratification is:
Number of Patients
The study will recruit 396 patients (198 receiving XT and 198 receiving PT) to detect a minimum 10% reduction in pulmonary complications.
Diagnosis & main inclusion criteria
Newly diagnosed patients with squamous cell carcinoma or adenocarcinoma of the esophagus, having resectable loco-regional disease without distant metastases and fulfilling the criteria for curatively intended nCPT or nCXT.
Treatment
nCPT and nCXT consist of weekly carboplatin and paclitaxel for 5 weeks, following the CROSS trial. The radiation dose will be either 41.4 Gy in 23 fractions or 50.4 Gy in 28 fractions. The pragmatic approach with respect to dose level will satisfy different clinical preferences and national guideline recommendations, and will thus allow broad trial acceptance across European institutions, will result in optimal accrual, and will promote subsequent widespread translation of results into clinical practice.
Primary endpoint
The primary outcome is the incidence of pulmonary complications during and following nCPT or nCXT and surgery. The proportion of patients with pulmonary complications will be compared between the arms. Complications are scored following CTCAE v5.0, grade ≥ 2 from nCPT or nCXT start until surgery & following Clavien-Dindo, grade ≥ 2 from surgery to 90 days after surgery. For patients not undergoing surgery, complications are scored within 90 days after completed nCPT or nCXT following CTCAE v5.0, grade ≥ 2.
Statistical methods
The primary comparisons will be analyzed according to the principle of intention to treat. All comparisons will be 2-sided and performed at the 5% level of significance. Pre-specified subgroups in which treatments will be compared: tumor location (esophageal versus gastroesophageal junction), histopathology (non-squamous versus squamous cell carcinoma), age (<75 years versus >75 years), sex, RT dose level (41.4Gy vs 50Gy), surgical treatment or not, surgical technique (as by stratification). Analyses will include tests of interaction. A full statistical analysis plan will be produced and will be approved by the Trial Steering Committee prior to unblinding of the study results. Analysis of the primary endpoint is planned 8 months after randomization of the final patient, and the primary trial outcome will be reported shortly thereafter. Further analyses will be conducted one, three and five years after the last patient has been included. Data will be updated up to 15 years after the last inclusion in the study.