To ensure consistent radiotherapy treatment in the Pan-European multicenter center setting, a comprehensive consensus guideline for planning the XT and PT has been designed. The guideline aims to handle the potential uncertainty in the delivery of PT compared with XT, and strict criteria for robust planning, handling of breathing motion, daily verification of treatment position and anatomical changes will be enforced. This includes mandatory treatment adaptation in case of dose deterioration.
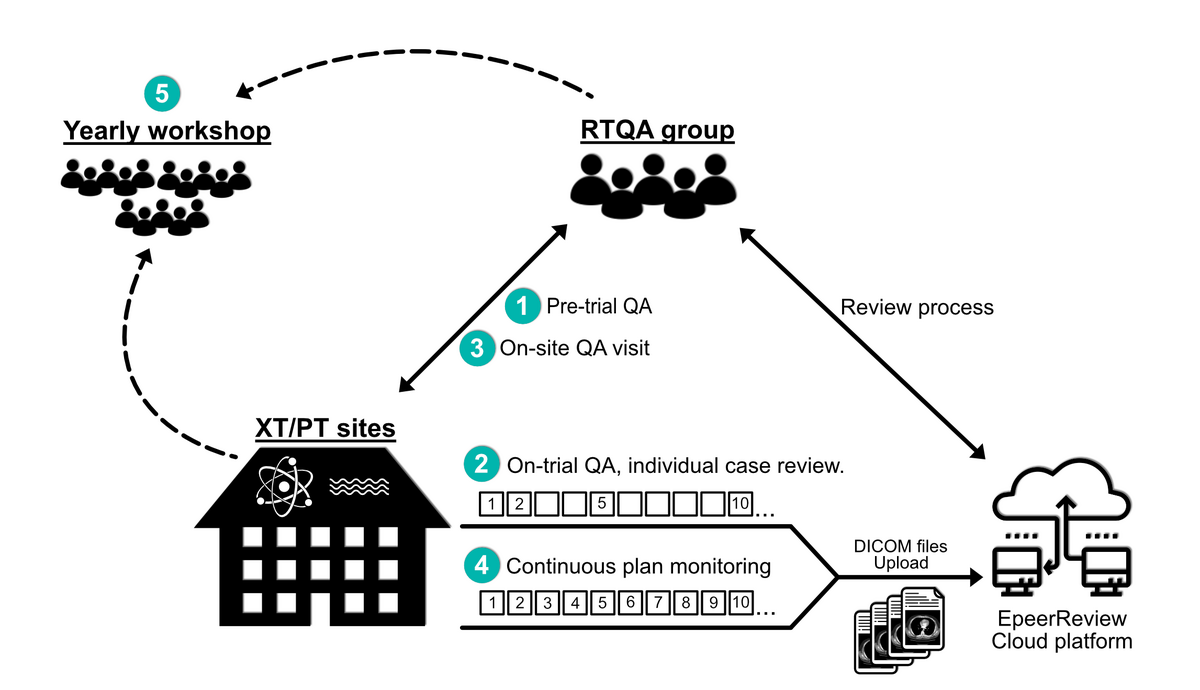
The RTQA program consists of five elements:
- Pre-trial QA consists of an XT/PT facility questionnaire, a dosimetry audit, a plan for handling breakdown, five delineation benchmark cases, and four treatment planning benchmark cases.
- On-trial QA consisting of prospective individual case review (ICR) by uploading DICOM files to ePeerReview:
- The first two patients enrolled in the trial at each center.
- ICR of every fifth patient after the first two.
- Independent dose calculation for all patients
- Plan check at the accelerator for all patients
- An on-site QA visit when a center has included the first two patients.
- Discuss scanning, delineation, treatment planning, treatment delivery, image guidance, and planned adaptation.
- Continuous XT/PT treatment plan monitoring. All dual treatment plans must be uploaded in an anonymized form to the central dose plan bank where the plans will be continuously monitored for adherence to the XT/PT guidelines. No strict time demand is set for continuous monitoring. Monitoring of plans will be used to check for variations of constraints and used for feedback to the centers. Further, the continuous QA program will monitor the difference in dose to organs at risk in the two arms. If variations in the dose difference potentially affect the primary endpoint power calculation, the QA group will notify the Chief Investigators.
- Yearly workshop. All centers will discuss ICRs, technical solutions, scientific work
The complete PROTECT RTQA guideline can be requested at rtqa_protect@rm.dk.